To start off,
Popular Mechanics (February 1968)
“The Amazing Rolamite — It Opens the Door for 1000 Inventions”
by
Norman Carlisle
One night in September 1966, a lean young, sandy-haired engineer named Donald Wilkes went into his garage workshop in Albuquerque NM to try an idea. What came out several hours later has been hailed as the first truly elementary mechanical invention of the 20th century.
Dubbed the Rolamite, it’s an almost frictionless bearing with countless applications in modern devices ranging from toasters to space vehicles. Engineers say it will take its place alongside the wheel, lever, and spring as a fundamental discovery of major significance.
Basically, the Rolamite consists of two rollers held in a track on opposite sides of an S-shaped band of springy metal, the rollers glide effortlessly in the track because the band moves with them as they roll along. Since the band and rollers are both moving at the same speed, there is no slip or drag between them and therefore virtually no friction. The device is so versatile it can function as a switch, a valve, a pump, a fuse, a thermostat, a force amplifier, a clutch, a speed changer, a brake, a pressure-sensing control, a solenoid, a fire alarm a — you name it and it’ll do it.
How could such a fundamental principle remain so long undiscovered? That was the first question I tossed at Donald Wilkes as I interviewed him recently in his equipment-crammed laboratory at Sandia Corp., the nuclear weaponry development center that Western Electric runs for the AEC.
“It’s hard to believe”, answered the 37-year-old inventor, who has been an avid PM reader since he was a boy. “The amazing thing is that a caveman has all the materials for making a Rolamite. Logs could have served for rollers and vines for the bands.”
So how had Wilkes come to invent the Rolamite? In the course of his missile work at Sandia, he had tinkered together a suspension system that particularly intrigued him. It consisted of a flexible metal band fastened in an S shape between two parallel surfaces. It was responsive to movements, all right — too responsive. “Wiggly and wobbly”, Wilkes describes it.
On that now-momentous night, Wilkes was relaxing in his living room when it hit him. How about putting rollers in the curves of the S? He jumped up and rushed out to his workshop. From his stock of scrap he fashioned a simple track and inserted a strip of beryllium copper he’d been carrying around in his pocket. From these components, he made the first Rolamite.
Now, Wilkes wondered, what would happen when he tipped the thing so that the rollers moved? Would the rollers slide along the curves of the band, or would the band move right along with the rollers with no slipping? Wilkes knew that if the band slipped he had nothing.
Again and again Wilkes tried it, his excitement growing. The rollers moved smoothly and the band went right with them. There was no detectable slip. The next morning he hurried to his lab to machine a more sophisticated model. Sensitive tests confirmed the observations made with the first crude model. There was no slipping and, therefore, little friction.
As development work went on, Wilkes and fellow researchers discovered that an almost infinite number of variations could be made by changing the shape, size and structure of the bands, rollers and tracks. Take the band, for example. So long as it’s under the same tension throughout its length, the rollers are stable at any point in the track. It takes just as much force to push them one way as the other way. But if you cut a slot in the band, you weaken it at that point, creating what is called a force bias — the rollers are made to “prefer” a particular point in the band.
To understand the effect of a slot in the band, think of the two loops of the S as springs that each exert a force against the other. Like a coiled watch spring, the band wants to lie flat and thus stores energy when it is forced to bend around the rollers.
When one of the loops is weakened by having a slot cut in it, the other loop overpowers it and “unwinds”, pulling the rollers with it. By cutting a long, tapered slot in the band, the rollers can be made to move the entire length of the track under their own power since the band becomes progressively weaker toward the widest end of the slot.
In a typical application, Wilkes visualizes a Rolamite with a slotted band to lick one common household annoyance — the leaky toilet valve. The leakiness usually results from the failure of the ball float and lever mechanism to generate enough pressure to close the water-supply valve. The force generated in a slotted Rolamite lever would close the valve with 30 times the strength of present valves.
Rollers can be Different Sizes
Wilkes’ first Rolamite used equal-sized rollers, but he soon found that one roller in a pair could be a giant, 10 or more times bigger than its companion. With rollers of different sizes, you get a remarkably simple speed changer that can be used in any number of mechanisms.
Perhaps the oddest discovery is that the rollers need not be round. Sandia researchers have tried triangular, hexagonal, oval and polygonal rollers. The basic principles of the Rolamite still apply just as with round rollers. The different shapes of rollers give the Rolamite many additional functions. For instance, a rectangular roller can be designed to lodge against a stop in a braking mechanism.
A lot of variations are possible in the track, too. For example, a track wider at one end makes the Rolamite a powerful force amplifier — energy is released when the rollers slip into the wider portion of the frame. This energy can actuate a variety of mechanisms, such as a firing pin or a switch.
There are other advantages, too. Many Rolamited devices would never need a drop of oil. Then there’s smoothness of operation. The steady, uniform operation of a Rolamite can take the jerks out of pop-up toasters, power sanders and a host of other devices.
There’s cost. Wilkes estimates that the Rolamite will actually reduce costs in 75% of its applications. The Rolamite does not require close tolerances, so they’re cheaper to make. Finally, there’s the all-around toughness. Extreme heat, cold or exposure to weather won’t affect Rolamite operation.
But, I wondered, doesn’t all that flexing of the band cause it to wear out eventually? Doesn’t metal fatigue cause it to break? Those were questions that bothered Sandia engineers too, at the beginning. Now they’ve quit worrying. The beryllium copper bands used in Rolamite have proved to be so sturdy that they show no sign of metal fatigue after 1,000,000 flexures. At that rate, the engineers figure, the band in a Rolamited home light switch operated 10 times a day would last 300 years. A Rolamited bathroom scale used five times a day would not wear out in 600 years.
You’ll never see the first applications of Rolamite because they’re tucked away in secret weaponry made by Sandia engineers. But hundreds of industries are embarking on crash programs to adapt the Rolamite.
Because Rolamite was developed with the help of tax dollars, it is available to the public. The Atomic Energy Commission will grant a royalty-free license for its manufacture to anyone interested. Wilkes himself now heads a new company set up to speed the Rolamite revolution along.
“We’ve just begun to scratch the surface”, Wilkes says. “Just wait until the independent inventors get going.”
How the Rolamite Works
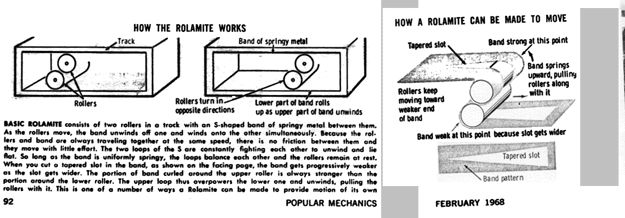
Basic Rolamite consists of two rollers in a track with an S-shaped band of springy metal between them. As the rollers move, the band unwinds off one and winds onto the other simultaneously. Because the rollers and band are always traveling together at the same speed, there is no friction between them and they move with little effort. The two loops of the S are constantly fighting each other to unwind and lie flat. So long as the band is uniformly springy, the loops balance each other and the rollers remain at rest. When you cut a tapered slot in the band, the band gets progressively weaker as the slot gets wider. The portion of band curled around the upper roller is always stronger than the portion around the lower roller. The upper loop thus overpowers the lower one and unwinds, pulling the rollers with it. This is one of a number of ways a Rolamite can be made to provide motion of its own.