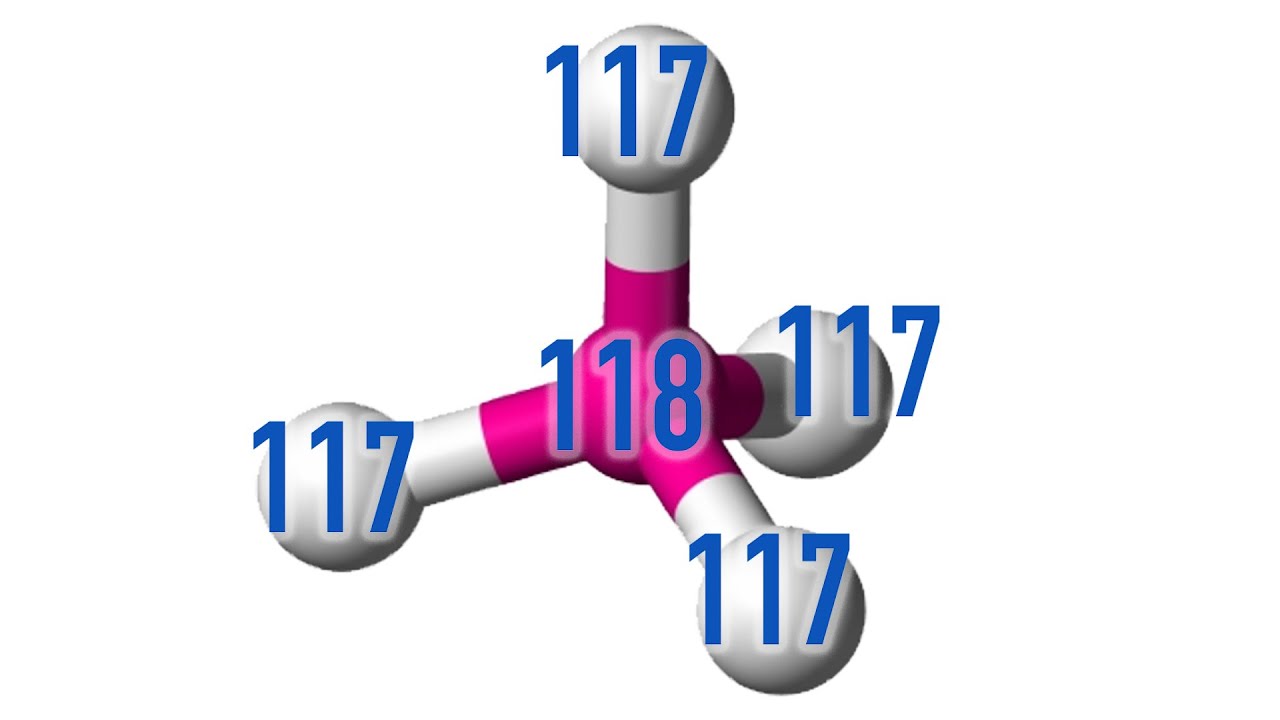
The research paper, “Relativistic effects for the superheavy reaction Og + 2Ts₂ → Og(Ts)₄: Dramatic relativistic effects for the atomization energy of Oganesson tetratennesside Og(Ts)₄ and the prediction of the existence of tetrahedral Og(Ts)₄”, uses relativistic computational chemistry to predict that a five-atom molecule formed of one atom of Oganesson (element 118) and four atoms of Tennesine (element 117) will be stable and have a tetrahedral form.
Don’t expect anybody to actually make even a single molecule of this stuff any time soon, however. The longest-lived known isotope of tennesine has a half-life of 51 milliseconds, while the only isotope of oganesson produced so far has a half-life of 690 microseconds.
The molecule is stable only due to the relativistic effects on its 586 electrons by the massive nuclei. Similar effects are responsible for the mellow yellow colour of gold.